Home >> Blog >> FDA Approved Elastomers and Sanitary Components
Supporting Manufacturers in Response to COVID-19
As industry mobilizes to support governments’ requests to fight COVID-19 and find ways to convert their production to manufacture much needed supplies, some of our customers are now producing Sanitizers and other FDA approved protection products in an effort to combat the COVID-19 virus.
If you are changing over your facilities to produce medical and sanitary supplies let Daemar be your source for FDA approved elastomers and urethane sealing components. This includes O-Rings, Seals, Cord, Extrusions and custom molded urethane parts.
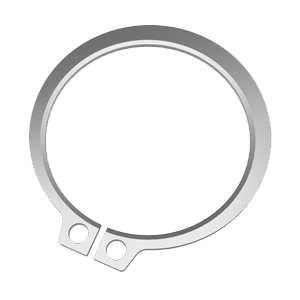
DMR retaining rings are readily available in stainless for FDA and sanitary applications.
Daemar components are found in many types of equipment operating in the demanding sanitary environments. Our seals, o-rings, bushings, retaining rings and shim are essential components in valves, pumps, and motors. Selecting the correct material and design for these components is crucial to the performance and longevity of your medical equipment.
Product Solutions for Sanitary Applications
Daemar’s range of product solutions for Medical, Sanitary and FDA applications include:
- X-Ray and Metal Detectable Seals – these industry specific parts can help eliminate product recall, lower product loss and stop distribution of contaminated product.
- Sanitary Gaskets – available in a range of FDA compliant elastomers, fluoroelastomers and Kalrez perfluoroelastomers.
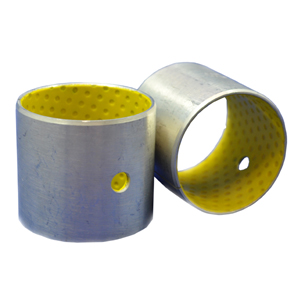
- FDA-USDA Self Lubricating Bearings – These bearings offer food process, pharmaceutical, packaging machinery and medical equipment engineers 3 off-the-shelf, FDA/USDA compliant linear bearing options.
- Braided Packing – FDA PTFE packing, interlock braid with a break-in lubricant, complies with the FDA and USDA requirements under Title 21 Food and Drugs
- PTFE Encapsulated Viton® O-rings – an o-ring that is virtually chemically inert and compression set resistant for use in harsh sealing environments.
- Caplugs Medical Parts – Instrument and Tip Protection, Connectors, Medical Packaging and Silicone Caps
These components are designed and engineered to perform in the harsh medical and pharmaceutical processing environment; the products must be resistant to the biological materials they are exposed to, they must also be able to withstand the regular cycle and chemicals of washdown. There is the added level of complexity created by the Government regulations and standards for products involved in medical applications.
To learn about selecting the correct products for your operating environment please refer to our Medical and Food Industry pages and contact your local Daemar Technical Sales Representative.